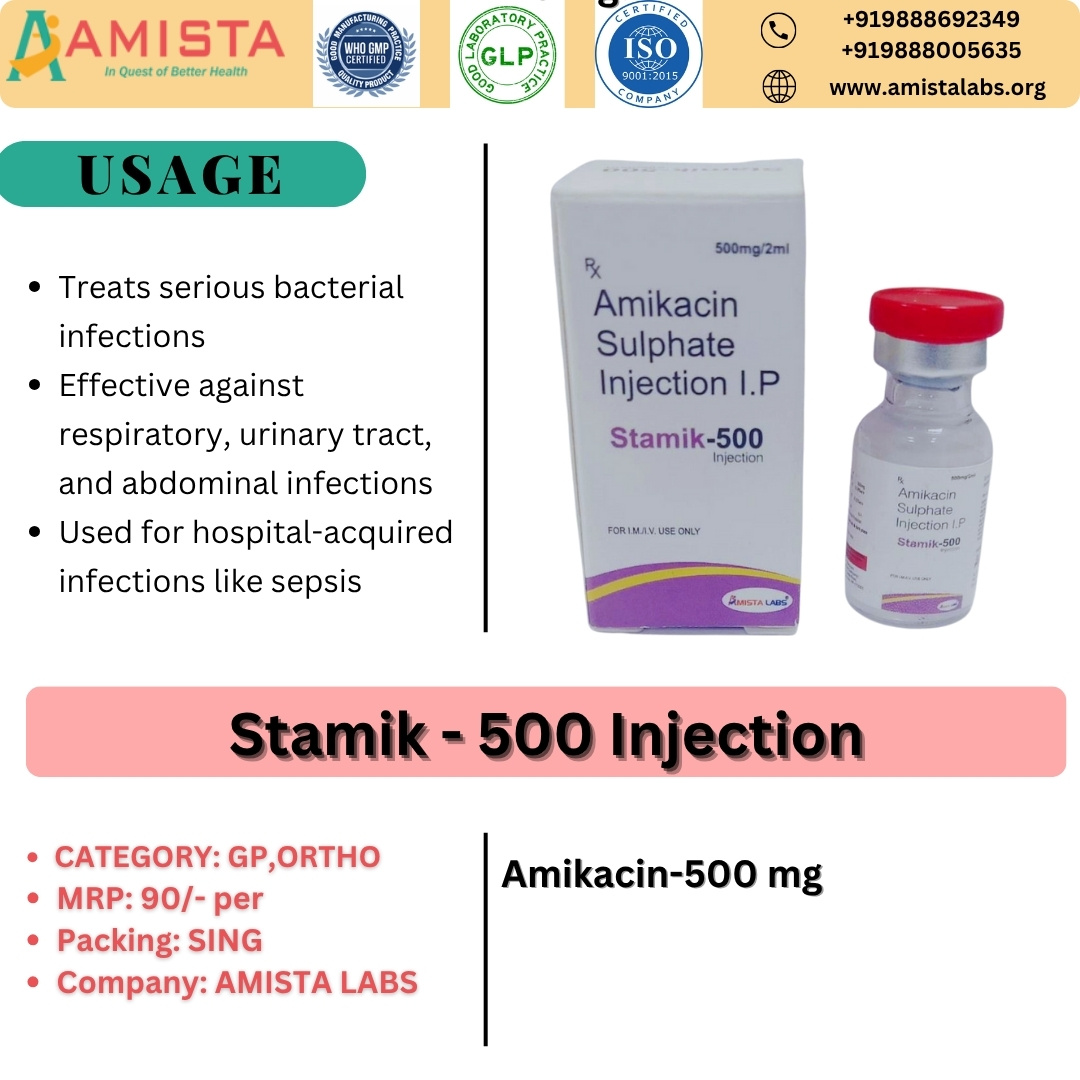
Stamik-500 Injection by Amista Labs is a high-quality antibiotic formulation designed for the effective management of serious bacterial infections. It contains Amikacin Sulphate Injection I.P. 500 mg, a potent aminoglycoside antibiotic known for its broad-spectrum activity against a wide range of Gram-negative and selected Gram-positive bacteria. Stamik-500 is commonly used in hospital and clinical settings where rapid and reliable antibacterial action is required.
Composition
Each 2 ml vial of Stamik-500 Injection contains:
The inclusion of preservatives ensures product stability, sterility, and safety throughout its shelf life.
Mechanism of Action
Amikacin works by inhibiting bacterial protein synthesis. It binds irreversibly to the 30S ribosomal subunit of susceptible bacteria, leading to the production of faulty proteins and ultimately causing bacterial cell death. Due to this bactericidal action, Stamik-500 provides fast and effective control over severe infections, even those caused by organisms resistant to other aminoglycosides.
Therapeutic Indications
Stamik-500 Injection is indicated in the treatment of serious infections caused by susceptible microorganisms, including:
-
Severe urinary tract infections
-
Lower respiratory tract infections
-
Septicemia and bloodstream infections
-
Bone and joint infections
-
Intra-abdominal infections
-
Skin and soft tissue infections
-
Post-operative and hospital-acquired infections
It is especially useful in treating infections caused by Pseudomonas aeruginosa, Escherichia coli, Klebsiella, Proteus, and other resistant Gram-negative bacteria.
Dosage and Administration
Physicians determine the dosage of Stamik-500 Injection based on the patient’s age, body weight, renal function, and severity of the infection. Healthcare professionals usually administer it via the intramuscular (IM) or intravenous (IV) route under strict medical supervision. In patients with impaired kidney function, physicians may adjust the dose to minimize the risk of toxicity.
Safety and Precautions
As with other aminoglycosides, healthcare professionals carefully monitor renal function and hearing during therapy. Physicians use Stamik-500 cautiously in patients with kidney disorders, dehydration, or pre-existing hearing problems. Clinicians avoid concomitant use with other nephrotoxic or ototoxic drugs unless absolutely necessary.
Storage Conditions
Store Stamik-500 Injection in a cool, dry place away from direct light. Do not freeze. Keep out of reach of children. Use the injection only if the solution is clear and free from visible particles.


 Users Today : 7
Users Today : 7 Users Yesterday : 24
Users Yesterday : 24 Users This Month : 986
Users This Month : 986 Users This Year : 2164
Users This Year : 2164
